Comments (continue).
BSDM (updated to strong buy)
October 19th, 2010 by BiotechInvest
This company has innovative and breakthrough technology for cancer treatment: microwave-mediated cancer hyperthermia and tumor ablation. Recently BSDM got FDA approval for next generation equipment. The company is potential buyout target.
Just check last company news:
October 14, 2010
BSD Launches MicroThermX Microwave Ablation System at CIRSE - Europe’s Largest Forum for Interventional Radiology Products
October 11, 2010
Cancer Treatment Centers of America Purchases BSD-2000 Hyperthermia System from BSD Medical
October 7, 2010
BSD Medical Reports Published Article Documents Rationale for Increased use of Hyperthermia
September 29, 2010
BSD Medical Obtains Patent on Innovative Design for Heat Therapy Devices
September 13, 2010
BSD Medical Reports Publication of Article on BSD’s Pioneering Technology to Improve Heat Therapy
September 9, 2010
BSD Medical’s China Distributor Orders Two BSD-2000 Hyperthermia Systems
September 1, 2010
BSD Medical Reports Receipt of Safety Certification for MicroThermX Microwave Ablation System
August 19, 2010
BSD Medical Announces Registered Direct Offering of Common Stock and Warrants in Aggregate Amount of $2.75 Million
August 18, 2010
BSD Medical Receives FDA 510(k) Clearance to Market the MicroThermX Microwave Ablation System
http://www.bsdmc.com/more-press.php
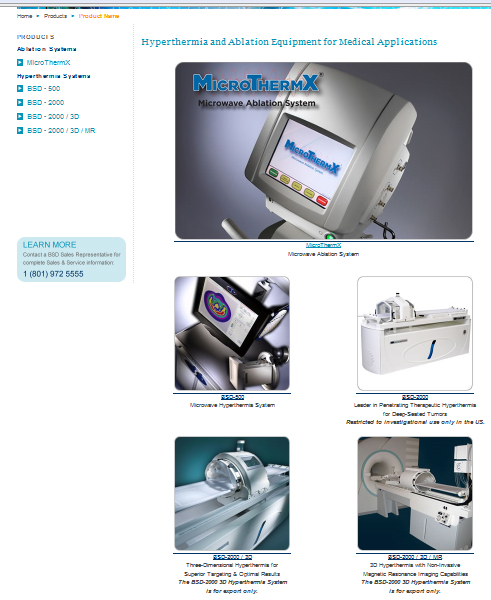
And look at BSDM products. It's real high-tech and 21th century equipment.
http://www.bsdmc.com/products.php
Strongly recommended for investment in 2010-2011. Low risk, high reward.
Disclosure: already have positions in BSDM, will increase it during next month.
October 20, 2010 by BiotechInvest
This is the really good news for BSDM. Only one question: why BSD-2000 is still in FDA pending approval during last several years?
BSD Medical Reports Publication in Internal Medicine News of Study Results Demonstrating Improved Survival for Sarcoma Cancer Patients Treated with Hyperthermia.
"Median disease-free survival was 32 months in the hyperthermia and chemotherapy group vs. 17 months in the chemotherapy only group." Again compare with DNDN Provenge results...
"The Phase III STBSG trial involved 341 patients who were treated at leading research centers in Europe and the United States and used the BSD-2000 Hyperthermia System to deliver hyperthermia combined with chemotherapy for the treatment of high-risk, soft-tissue sarcoma cancer patients."
http://finance.yahoo.com/news/BSD-Medical-Reports-bw-1244746566.html?x=0&.v=1
October 25, 2010 by BiotechInvest
Again we have very good news for BSDM
BSD Medical Announces Purchase of BSD-500 Hyperthermia System by University Medical Center of Göttingen, Germany
There are only 6 trade days before November 2nd, approximate earnings release of BSDM.
November 2, 2010 by BiotechInvest
According TD Ameritrade "the next earnings announcement from BSDM is expected the week of November 8, 2010."
BSDM reported 3rd quarter 2010 losses of $0.10 per share on July 15, 2010.
November 3, 2010 by BiotechInvest
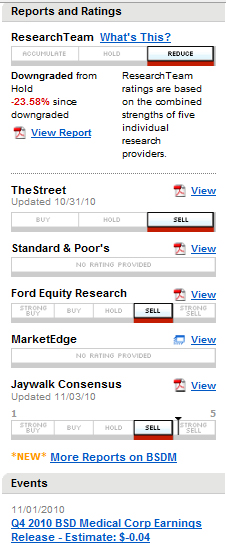
It seems like that for analysts any BSDM news are bad news. Read recent Street report here and Ford Equity Research here.
So, all analysts said "sell". OK, they did "perfect" technical analysis.
What about BSDM innovative science and technology that will allow to do very important breakthrough in cancer fighting? It seems like it's not their busyness...
I found recently published paper that supports idea that BSDM products, and especially BSD-2000 have a bright future in cancer treatment. PDF file of paper is here.
Oncol Rep. 2010 Sep;24(3):591-8.
Evaluation of systemic external microwave hyperthermia for treatment of pleural metastasis in orthotopic lung cancer model.
Motomura T, Ueda K, Ohtani S, Hansen E, Ji L, Ito K, Saito K, Sugita Y, Nosé Y.
Baylor College of Medicine, Michael E DeBakey Department of Surgery, Houston, TX 77030, USA. tmotomura@sbcglobal.net
Abstract
External microwave (EMW) hyperthermia system (2.45 GHz wave frequency) was evaluated by in vitro studies and in vivo pleural metastasis animal model. Three different non-small-cell lung cancer cells and normal fibroblast cells (control) were treated once a day for 3 days with the prototype EMW system applying mild (39 degrees C), moderate (43 degrees C), and severe (47 degrees C) hyperthermia. On Day-4, tested cells were retrieved and examined by apoptosis assay kit and Western blot analysis. Cancer cells treated with moderate hyperthermia showed significant apoptosis; yet no major damage was observed to normal fibroblast cells. Western blot analysis indicated cleavage on caspase-3, -9 and PARP. Also in the cell cycle analysis, increase of sub G0-G1 population was identified. After optimization of the heating intensity for in vivo environment, we created pleural metastatic animal model in 24 immune deficiency mice (male nu/nu mice) to evaluate inhibitory effect of systemic EMW hyperthermia for disseminated tumor growth. Out of 24 mice, 8 received mild and 8 received moderate hyperthermia, and remaining 8 were the no treatment control. Whole chest area of the experimental animals was irradiated 3 times a week for 2 weeks (total of 6 time irradiations). No significant adverse event was observed including abnormal weight loss, skin burn, ulceration, and death. Metastasized tumors around the pleura and chest cavity were 75% reduced in size and weight compared to non-treated control group. Harvested tumors were stained and TUNEL assay demonstrated significant apoptosis in a moderate hyperthermia group. The EMW hyperthermia system may be possible alternative tool as a systemic hyperthermia therapy in severely advanced lung cancer patients. Further study is necessary to determine device safeness, efficacy, and synergistic effect to other possible combination therapies.
Again repeat it: "Metastasized tumors around the pleura and chest cavity were 75% reduced in size and weight compared to non-treated control group."
"non-small-cell lung cancer"
"More people die from lung cancer than any other type of cancer, the Centers for Disease Control and Prevention notes. In 2006, it accounted for more deaths than breast cancer, prostate cancer and colon cancer combined. Just over 200,000 people were diagnosed with lung cancer in 2006, and nearly 160,000 died from lung cancer that year."
Authors said in Discussion:
"However,a large animal involves much thicker subcutaneous muscle and fat tissues,larger volume of the organs compared to small animals (i.e.mice);hence it is difficult for the irradiated microwave beam to reach the targeted cancer tumors,which were generally developed at deep and multiple anatomical locations.Further investigation using different wave-length and heating regimen will be needed prior to large animal experiments."
Come on, guys this equipment is already invented by BSDM. It's BSD-2000 that is still under FDA review at least from 2006.
I think that this paper results are very important because authors showed that it's not necessarily to ablate cancer tissue, but better use several short time hyperthermia treatments. Cancer cells dividing very often and it's easy to catch them at a phase called G1.
The G1 phase arrest is accompanied by apoptosis. Thus, cancer cells will be killed by apoptosis (the process of programmed cell death).
November 04, 2010 By BiotechInvest
Hudson Valley Oncology Purchases BSD-500 Hyperthermia System from BSD Medical
BSD Medical Corporation (NASDAQ:BSDM - News) (the “Company” or “BSD”) announced today that Hudson Valley Oncology Associates has purchased the BSD-500 Hyperthermia System (BSD-500), and will provide hyperthermia services at its cancer center, PROS, Professional Radiation Oncology Services, Poughkeepsie, N.Y. These radiation oncology partners have been working together as cancer care providers for over 20 years, and they specialize in providing state-of-the-science approaches to diagnosing and treating cancer. PROS offers an exceptional level of excellence in cancer care to the Mid-Hudson, New York, area.
November 09, 2010 by BiotechInvest
I'm sorry for previously published information:
November 2, 2010 by BiotechInvest
According TD Ameritrade "the next earnings announcement from BSDM is expected the week of November 8, 2010."
I called BSDM today and they said that the earning release will be published at the end of November i.e. November 28-29th.
Both TD Ameritrade and Yahoo took November 8th as earning release date from nowhere, they never contacted BSDM.
I called and talked with Dennis.
Dennis P. Gauger, CPA
Chief Financial Officer
investor@bsdmc.com
BSD Medical Corporation
2188 West 2200 South
Salt Lake City, Utah 84119
Phone: (801) 924-7862
Fax: (801) 924-7863
Today BSDM pps is much closer to real company pps. I think that after all known news (today too) and earning release we will see real pps i.e. $10-12.
November 15, 2010 by BiotechInvest
Were today news bad or good for BSDM?
Two BSDM institutional investors bought 1,750,000 shares of BSDM ($5.97 per share). In addition, warrants to purchase 875,000 shares of common stock in the aggregate will be issued to the investors. The warrants are exercisable beginning six months and one day after closing, expire 5 years after becoming exercisable and have an exercise price of $7.73 per share.
They bought stock that has 52wk Range: $0.86 - 7.40 for $5.97 per share!!!
Do somebody really think that these news are bad news for BSDM? Funds bought almost 2M and want more! I'm sure that they know exact BSDM pps and they want at least 100% gain in nearest future. Well, they will get it soon. May be even before Christmas...
Movie explaining how hyperthermia works here.
November 29, 2010 by BiotechInvest
As usually any BSDM news are "interpreted" by someone as a bad news.
Form 10-K was not so impressive, but just read this:
"Subsequent to the August 31, 2010, year-end period of the Annual Report, we have received purchase orders for seven hyperthermia systems totaling approximately $2.4 million."
It means that during Q IV BSDM got orders for new equipment that is equivalent to company annual sales. And these orders for most advanced BSDM equipment: BSD-2000 and BSD-500.
Any way, some funds that are practicing "short trades" used these news to initiate BSDM sell off today. "Never fight with market" so I trim my BSDM positions today (50%). I'll keep residue for a long. I hope that good news are coming.
December 14, 2010 by BiotechInvest
Today news are great for BSDM. However, it seems like that pps spike was driven by shorts covering. I sold all BSDM positions today to avoid previous losses. I'll monitor BSDM and may be will invest in 2011 (before next earning release).
12.18.2011 by BiotechInvest
BSD-2000 got FDA HDE Marketing Approval but BSDM pps is still in $2 range...
And doctors are still using a primitive approach to "Boiling breast tumours kills them..."
"Doctors have developed a new treatment that kills breast cancer cells by ‘cooking’ them — and it works in just minutes.
The procedure uses a targeted electrical current to heat tumours to 70-90c (160-190f).
Studies show this kills cancer cells in ten minutes — the woman can then go home or back to work shortly afterwards.
The treatment, known as Preferential Radio-Frequency Ablation and carried out under local anaesthetic, is being pioneered by doctors at the Karolinska Institute in Sweden.
Doctors use ultrasound to guide a thin, needle-like electrode into the middle of the tumour in the breast.
An electric current is then put through the electrode to heat the tumour to up to 90 c, killing it while leaving the surrounding tissue unharmed.
Come on, BSD-2000 can do it without any needle-like electrode.
Afterwards, the dead tissue is left inside the body and turns into a harmless scar. Only one treatment is needed.
Dr Karin Leifland, the radiologist overseeing the research, says the technology could become an alternative to surgery for women with early stage breast cancer.
Similar treatments using radio-frequency ablation are already being used to treat kidney, liver and bone cancers.
‘It is like boiling an egg,’ explains Dr Leifland.
‘The tumour is heated to such an extent that the cancer cells are killed off, while leaving the surrounding tissue unharmed.
‘The treatment is suitable mainly for women with tumours which are smaller than 2 cm and are contained in a single lump.
'There is no pain or scar afterwards and within minutes of the treatment, women can leave the hospital and go home or back to work.’
Any way these results said that the radio-frequency ablation of cancer has a bright future. But the U.S. health regulator approved BSD-2000 only to treat cervical cancer.
HDE approval is given to a company after it demonstrates its product's safety and probable benefit for the treatment of a disease affecting fewer than 4,000 people in the United States every year.
In 2010, an estimated 12,200 women in the United States were diagnosed with cervical cancer, and an estimated 4,210 died of the disease. It is estimated that about $1.4 billion is spent in the United States each year on cervical cancer treatment, according to the company.
BSD Medical Signs Exclusive Distribution Agreement with CyberKnife Korea for Distribution of BSD’s Hyperthermia Systems in South Korea
SALT LAKE CITY -- December 12, 2011 -- BSD Medical Corporation (NASDAQ:BSDM) (Company or BSD) (www.BSDMedical.com), a leading provider of medical systems that utilize heat therapy to treat cancer, announced today that the Company has signed an exclusive agreement with CyberKnife Korea (CKK) for the sale and distribution of BSD’s hyperthermia products in South Korea. CKK is a premier distributor of sophisticated medical devices in South Korea and represents a number of major medical device companies. CKK is a leading distributor of oncology products in South Korea and has established strong relationships with radiation oncologists throughout the country. As part of the agreement, CKK is required to purchase a minimum number of hyperthermia systems from BSD each year. BSD will initially focus on the regulatory approval for and distribution of the BSD-500 Hyperthermia System and then expand distribution to include the BSD-2000 Hyperthermia System.
South Korea ranks as one of the world’s leading economies, with a population of 61 million and overall GDP among the top 15 in the world. As a result, much of the population expects a high level of medical care. The South Korea medical equipment market is focused on high-end, innovative technology with a strong reliance on imported products. The 2010 oncology market in South Korea was approximately US $840 million.
“We are excited to establish a business relationship with one of the leading oncology product distributors in Korea,” stated Harold R. Wolcott, President of BSD Medical. “The Asia Pacific countries are becoming increasingly important as a market and could represent up to 40% of the global health care market by 2015. The receipt of the HDE marketing approval for the BSD-2000 will expand the market in Asia for BSD’s hyperthermia products. BSD is committed to establishing strong partnerships in the Asia Pacific countries that will result in increased product sales in this region.”
Conclusions: BSDM pps is artificially understated and $66M cap can't be real for biotech with such great and innovative products. Current pps ($2.23) is a good for entry. I'll buy BSDM next week.
October 19th, 2010 by BiotechInvest
This company has innovative and breakthrough technology for cancer treatment: microwave-mediated cancer hyperthermia and tumor ablation. Recently BSDM got FDA approval for next generation equipment. The company is potential buyout target.
Just check last company news:
October 14, 2010
BSD Launches MicroThermX Microwave Ablation System at CIRSE - Europe’s Largest Forum for Interventional Radiology Products
October 11, 2010
Cancer Treatment Centers of America Purchases BSD-2000 Hyperthermia System from BSD Medical
October 7, 2010
BSD Medical Reports Published Article Documents Rationale for Increased use of Hyperthermia
September 29, 2010
BSD Medical Obtains Patent on Innovative Design for Heat Therapy Devices
September 13, 2010
BSD Medical Reports Publication of Article on BSD’s Pioneering Technology to Improve Heat Therapy
September 9, 2010
BSD Medical’s China Distributor Orders Two BSD-2000 Hyperthermia Systems
September 1, 2010
BSD Medical Reports Receipt of Safety Certification for MicroThermX Microwave Ablation System
August 19, 2010
BSD Medical Announces Registered Direct Offering of Common Stock and Warrants in Aggregate Amount of $2.75 Million
August 18, 2010
BSD Medical Receives FDA 510(k) Clearance to Market the MicroThermX Microwave Ablation System
http://www.bsdmc.com/more-press.php
And look at BSDM products. It's real high-tech and 21th century equipment.
http://www.bsdmc.com/products.php
Strongly recommended for investment in 2010-2011. Low risk, high reward.
Disclosure: already have positions in BSDM, will increase it during next month.
October 20, 2010 by BiotechInvest
This is the really good news for BSDM. Only one question: why BSD-2000 is still in FDA pending approval during last several years?
BSD Medical Reports Publication in Internal Medicine News of Study Results Demonstrating Improved Survival for Sarcoma Cancer Patients Treated with Hyperthermia.
"Median disease-free survival was 32 months in the hyperthermia and chemotherapy group vs. 17 months in the chemotherapy only group." Again compare with DNDN Provenge results...
"The Phase III STBSG trial involved 341 patients who were treated at leading research centers in Europe and the United States and used the BSD-2000 Hyperthermia System to deliver hyperthermia combined with chemotherapy for the treatment of high-risk, soft-tissue sarcoma cancer patients."
http://finance.yahoo.com/news/BSD-Medical-Reports-bw-1244746566.html?x=0&.v=1
October 25, 2010 by BiotechInvest
Again we have very good news for BSDM
BSD Medical Announces Purchase of BSD-500 Hyperthermia System by University Medical Center of Göttingen, Germany
There are only 6 trade days before November 2nd, approximate earnings release of BSDM.
November 2, 2010 by BiotechInvest
According TD Ameritrade "the next earnings announcement from BSDM is expected the week of November 8, 2010."
BSDM reported 3rd quarter 2010 losses of $0.10 per share on July 15, 2010.
November 3, 2010 by BiotechInvest
It seems like that for analysts any BSDM news are bad news. Read recent Street report here and Ford Equity Research here.
So, all analysts said "sell". OK, they did "perfect" technical analysis.
What about BSDM innovative science and technology that will allow to do very important breakthrough in cancer fighting? It seems like it's not their busyness...
I found recently published paper that supports idea that BSDM products, and especially BSD-2000 have a bright future in cancer treatment. PDF file of paper is here.
Oncol Rep. 2010 Sep;24(3):591-8.
Evaluation of systemic external microwave hyperthermia for treatment of pleural metastasis in orthotopic lung cancer model.
Motomura T, Ueda K, Ohtani S, Hansen E, Ji L, Ito K, Saito K, Sugita Y, Nosé Y.
Baylor College of Medicine, Michael E DeBakey Department of Surgery, Houston, TX 77030, USA. tmotomura@sbcglobal.net
Abstract
External microwave (EMW) hyperthermia system (2.45 GHz wave frequency) was evaluated by in vitro studies and in vivo pleural metastasis animal model. Three different non-small-cell lung cancer cells and normal fibroblast cells (control) were treated once a day for 3 days with the prototype EMW system applying mild (39 degrees C), moderate (43 degrees C), and severe (47 degrees C) hyperthermia. On Day-4, tested cells were retrieved and examined by apoptosis assay kit and Western blot analysis. Cancer cells treated with moderate hyperthermia showed significant apoptosis; yet no major damage was observed to normal fibroblast cells. Western blot analysis indicated cleavage on caspase-3, -9 and PARP. Also in the cell cycle analysis, increase of sub G0-G1 population was identified. After optimization of the heating intensity for in vivo environment, we created pleural metastatic animal model in 24 immune deficiency mice (male nu/nu mice) to evaluate inhibitory effect of systemic EMW hyperthermia for disseminated tumor growth. Out of 24 mice, 8 received mild and 8 received moderate hyperthermia, and remaining 8 were the no treatment control. Whole chest area of the experimental animals was irradiated 3 times a week for 2 weeks (total of 6 time irradiations). No significant adverse event was observed including abnormal weight loss, skin burn, ulceration, and death. Metastasized tumors around the pleura and chest cavity were 75% reduced in size and weight compared to non-treated control group. Harvested tumors were stained and TUNEL assay demonstrated significant apoptosis in a moderate hyperthermia group. The EMW hyperthermia system may be possible alternative tool as a systemic hyperthermia therapy in severely advanced lung cancer patients. Further study is necessary to determine device safeness, efficacy, and synergistic effect to other possible combination therapies.
Again repeat it: "Metastasized tumors around the pleura and chest cavity were 75% reduced in size and weight compared to non-treated control group."
"non-small-cell lung cancer"
"More people die from lung cancer than any other type of cancer, the Centers for Disease Control and Prevention notes. In 2006, it accounted for more deaths than breast cancer, prostate cancer and colon cancer combined. Just over 200,000 people were diagnosed with lung cancer in 2006, and nearly 160,000 died from lung cancer that year."
Authors said in Discussion:
"However,a large animal involves much thicker subcutaneous muscle and fat tissues,larger volume of the organs compared to small animals (i.e.mice);hence it is difficult for the irradiated microwave beam to reach the targeted cancer tumors,which were generally developed at deep and multiple anatomical locations.Further investigation using different wave-length and heating regimen will be needed prior to large animal experiments."
Come on, guys this equipment is already invented by BSDM. It's BSD-2000 that is still under FDA review at least from 2006.
I think that this paper results are very important because authors showed that it's not necessarily to ablate cancer tissue, but better use several short time hyperthermia treatments. Cancer cells dividing very often and it's easy to catch them at a phase called G1.
The G1 phase arrest is accompanied by apoptosis. Thus, cancer cells will be killed by apoptosis (the process of programmed cell death).
November 04, 2010 By BiotechInvest
Hudson Valley Oncology Purchases BSD-500 Hyperthermia System from BSD Medical
BSD Medical Corporation (NASDAQ:BSDM - News) (the “Company” or “BSD”) announced today that Hudson Valley Oncology Associates has purchased the BSD-500 Hyperthermia System (BSD-500), and will provide hyperthermia services at its cancer center, PROS, Professional Radiation Oncology Services, Poughkeepsie, N.Y. These radiation oncology partners have been working together as cancer care providers for over 20 years, and they specialize in providing state-of-the-science approaches to diagnosing and treating cancer. PROS offers an exceptional level of excellence in cancer care to the Mid-Hudson, New York, area.
November 09, 2010 by BiotechInvest
I'm sorry for previously published information:
November 2, 2010 by BiotechInvest
According TD Ameritrade "the next earnings announcement from BSDM is expected the week of November 8, 2010."
I called BSDM today and they said that the earning release will be published at the end of November i.e. November 28-29th.
Both TD Ameritrade and Yahoo took November 8th as earning release date from nowhere, they never contacted BSDM.
I called and talked with Dennis.
Dennis P. Gauger, CPA
Chief Financial Officer
investor@bsdmc.com
BSD Medical Corporation
2188 West 2200 South
Salt Lake City, Utah 84119
Phone: (801) 924-7862
Fax: (801) 924-7863
Today BSDM pps is much closer to real company pps. I think that after all known news (today too) and earning release we will see real pps i.e. $10-12.
November 15, 2010 by BiotechInvest
Were today news bad or good for BSDM?
Two BSDM institutional investors bought 1,750,000 shares of BSDM ($5.97 per share). In addition, warrants to purchase 875,000 shares of common stock in the aggregate will be issued to the investors. The warrants are exercisable beginning six months and one day after closing, expire 5 years after becoming exercisable and have an exercise price of $7.73 per share.
They bought stock that has 52wk Range: $0.86 - 7.40 for $5.97 per share!!!
Do somebody really think that these news are bad news for BSDM? Funds bought almost 2M and want more! I'm sure that they know exact BSDM pps and they want at least 100% gain in nearest future. Well, they will get it soon. May be even before Christmas...
Movie explaining how hyperthermia works here.
November 29, 2010 by BiotechInvest
As usually any BSDM news are "interpreted" by someone as a bad news.
Form 10-K was not so impressive, but just read this:
"Subsequent to the August 31, 2010, year-end period of the Annual Report, we have received purchase orders for seven hyperthermia systems totaling approximately $2.4 million."
It means that during Q IV BSDM got orders for new equipment that is equivalent to company annual sales. And these orders for most advanced BSDM equipment: BSD-2000 and BSD-500.
Any way, some funds that are practicing "short trades" used these news to initiate BSDM sell off today. "Never fight with market" so I trim my BSDM positions today (50%). I'll keep residue for a long. I hope that good news are coming.
December 14, 2010 by BiotechInvest
Today news are great for BSDM. However, it seems like that pps spike was driven by shorts covering. I sold all BSDM positions today to avoid previous losses. I'll monitor BSDM and may be will invest in 2011 (before next earning release).
12.18.2011 by BiotechInvest
BSD-2000 got FDA HDE Marketing Approval but BSDM pps is still in $2 range...
And doctors are still using a primitive approach to "Boiling breast tumours kills them..."
"Doctors have developed a new treatment that kills breast cancer cells by ‘cooking’ them — and it works in just minutes.
The procedure uses a targeted electrical current to heat tumours to 70-90c (160-190f).
Studies show this kills cancer cells in ten minutes — the woman can then go home or back to work shortly afterwards.
The treatment, known as Preferential Radio-Frequency Ablation and carried out under local anaesthetic, is being pioneered by doctors at the Karolinska Institute in Sweden.
Doctors use ultrasound to guide a thin, needle-like electrode into the middle of the tumour in the breast.
An electric current is then put through the electrode to heat the tumour to up to 90 c, killing it while leaving the surrounding tissue unharmed.
Come on, BSD-2000 can do it without any needle-like electrode.
Afterwards, the dead tissue is left inside the body and turns into a harmless scar. Only one treatment is needed.
Dr Karin Leifland, the radiologist overseeing the research, says the technology could become an alternative to surgery for women with early stage breast cancer.
Similar treatments using radio-frequency ablation are already being used to treat kidney, liver and bone cancers.
‘It is like boiling an egg,’ explains Dr Leifland.
‘The tumour is heated to such an extent that the cancer cells are killed off, while leaving the surrounding tissue unharmed.
‘The treatment is suitable mainly for women with tumours which are smaller than 2 cm and are contained in a single lump.
'There is no pain or scar afterwards and within minutes of the treatment, women can leave the hospital and go home or back to work.’
Any way these results said that the radio-frequency ablation of cancer has a bright future. But the U.S. health regulator approved BSD-2000 only to treat cervical cancer.
HDE approval is given to a company after it demonstrates its product's safety and probable benefit for the treatment of a disease affecting fewer than 4,000 people in the United States every year.
In 2010, an estimated 12,200 women in the United States were diagnosed with cervical cancer, and an estimated 4,210 died of the disease. It is estimated that about $1.4 billion is spent in the United States each year on cervical cancer treatment, according to the company.
BSD Medical Signs Exclusive Distribution Agreement with CyberKnife Korea for Distribution of BSD’s Hyperthermia Systems in South Korea
SALT LAKE CITY -- December 12, 2011 -- BSD Medical Corporation (NASDAQ:BSDM) (Company or BSD) (www.BSDMedical.com), a leading provider of medical systems that utilize heat therapy to treat cancer, announced today that the Company has signed an exclusive agreement with CyberKnife Korea (CKK) for the sale and distribution of BSD’s hyperthermia products in South Korea. CKK is a premier distributor of sophisticated medical devices in South Korea and represents a number of major medical device companies. CKK is a leading distributor of oncology products in South Korea and has established strong relationships with radiation oncologists throughout the country. As part of the agreement, CKK is required to purchase a minimum number of hyperthermia systems from BSD each year. BSD will initially focus on the regulatory approval for and distribution of the BSD-500 Hyperthermia System and then expand distribution to include the BSD-2000 Hyperthermia System.
South Korea ranks as one of the world’s leading economies, with a population of 61 million and overall GDP among the top 15 in the world. As a result, much of the population expects a high level of medical care. The South Korea medical equipment market is focused on high-end, innovative technology with a strong reliance on imported products. The 2010 oncology market in South Korea was approximately US $840 million.
“We are excited to establish a business relationship with one of the leading oncology product distributors in Korea,” stated Harold R. Wolcott, President of BSD Medical. “The Asia Pacific countries are becoming increasingly important as a market and could represent up to 40% of the global health care market by 2015. The receipt of the HDE marketing approval for the BSD-2000 will expand the market in Asia for BSD’s hyperthermia products. BSD is committed to establishing strong partnerships in the Asia Pacific countries that will result in increased product sales in this region.”
Conclusions: BSDM pps is artificially understated and $66M cap can't be real for biotech with such great and innovative products. Current pps ($2.23) is a good for entry. I'll buy BSDM next week.