Comments (continue).
September 30th, 2010 by BiotechInvest (November 01, 2011 updated to Strong Buy) 02.03.2012 updated to hold or sell
Very short comment about AMLN (buy) Bydureon (PDUFA Oct. 22)
BYDUREON is a once-weekly formulation of exenatide, the active ingredient in BYETTA® (exenatide) injection, which has been available in the U.S. since June 2005 and is used in approximately 60 countries worldwide to improve glycemic control in adults with type 2 diabetes. This new formulation based on based on Alkermes' proprietary Medisorb technology for long-acting medications.
This technology was acquire by Alkermes together with Medisorb Technologies International, L.P., a leader in the development of injectable controlled release drug delivery technologies.
"In this technology, medication is encapsulated in microspheres made of a medical-grade polymer called polylactide co-glycolide (PLG). Each microsphere is about one-tenth of a millimeter in size, roughly equivalent to the diameter of a human hair. PLG is a common, biodegradable polymer with a history of safe human use in sutures, bone plates and extended-release pharmaceuticals. Over time, the PLG polymer breaks down into lactic acid and glycolic acid, which are completely metabolized by the body and eliminated as carbon dioxide and water, thereby releasing the medication.
Upon injection, the microspheres begin to absorb water almost immediately, leading to a swelling of the microspheres. This process begins a phase in which a small amount of medication at or near the surface of the microspheres is released. Over time water slowly breaks down the polymer structure allowing medication to release, resulting in a sustained supply of medication. The polymer matrix eventually breaks down and is eliminated from the body as carbon dioxide and water."
Why FDA delayed this very comfortable for patients drug?
In the complete response letter there are no requests for new pre-clinical or clinical trials. Requests raised in the letter primarily relate to the finalization of the product labeling with accompanying Risk Evaluation and Mitigation Strategy (REMS) and clarification of existing manufacturing processes.
Almost same story was happened with Savient gout drug Krystexxa (pegloticase) http://www.redorbit.com/news/health/1730601/savient_pharmaceuticals_receives_complete_response_letter_from_us_food_and/index.html
Everybody know now that Krystexxa got an approval.
Conclusion: Bydureon is more safe in comparison with already approved Victoza that has next black box label:
In animal studies, Victoza® caused thyroid tumors—including thyroid cancer—in some rats and mice. It is not known whether Victoza® causes thyroid tumors or a type of thyroid cancer called medullary thyroid cancer (MTC) in people which may be fatal if not detected and treated early. Do not use Victoza® if you or any of your family members have a history of MTC or if you have Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). While taking Victoza®, tell your doctor if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer (http://www.victoza.com). So, the probability of Bydureon approval is close to 100 %.
Disclosure: already have AMLN in portfolio, will keep it through PDUFA (Oct. 22th).
October 20, 2010 by BiotechInvest
Unbelievable... It's the only one word I had when I read FDA CRL to AMLN.
"In the complete response letter the FDA requested a thorough QT (tQT) study with exposures of exenatide higher than typical therapeutic levels of BYDUREON"
Are they crazy or completely stupid? The exenatide single SC injection dose is 10 mcg, but weekly Bydureon single dose of extended release contains 0.8 mgof exenatide(i.e. 800 mcg of Exenatide).
Pharmacokinetics/Absorption of Byetta (exenatide)
Following SC administration to patients with type 2 diabetes, exenatide reaches median peak plasma concentrations in 2.1 h. The mean peak exenatide concentration (Cmax) was 211 pg/mL and overall mean area under the time-concentration curve (AUC0-inf) was 1036 pg•h/mL following SC administration of a 10-mcg dose of BYETTA. Exenatide exposure (AUC) increased proportionally over the therapeutic dose range of 5 mcg to 10 mcg. The Cmax values increased less than proportionally over the same range. Similar exposure is achieved with SC administration of BYETTA in the abdomen, thigh, or upper arm. Nonclinical studies have shown that exenatide is predominantly eliminated by glomerular filtration with subsequent proteolytic degradation. The mean apparent clearance of exenatide in humans is 9.1 L/h and the mean terminal half-life is 2.4 h. These pharmacokinetic characteristics of exenatide are independent of the dose.
http://www.rxlist.com/byetta-drug.htm
Well, what about exenatide plasma level after single weekly injection of BYDUREON? Single injection of BYDUREON may contains 0.8-2 mg of exenatide but in SR/CR (sustained/controlled release form).
I found in recent publication about BYDUREON next numbers:
"Steady-state plasma exenatide concentrations were observed by Week 8 of the study. For the evaluable pharmacokinetic population, geometric mean (90% confidence interval) steady-state plasma concentrations (pg/mL) were 81.2 (68.3-96.4) and 344.5 (256.5-462.7) with exenatide QW 0.8 mg (n=8) and exenatide QW 2.0 mg (n=5), respectively.
0.8 mg 2.0 mg
Day 1 Cmax (pg/mL) 64.3 (38.3-107.8) 137.3 (74.6-252.6)
AUC (0-8 hours) (pg*h/mL) 187.6 (133.7-263.3) 405.6 (278.4-590.8)
Cave,ss (pg/mL)c 81.2 (68.3-96.4) 344.5 (256.5-462.7)
"In the complete response letter the FDA requested a thorough QT (tQT) study with exposures of exenatide higher than typical therapeutic levels of BYDUREON"
So, if patient injected with single dose of twice-daily exenatide he has average Cmax 211 pg/mL and overall mean area under the time-concentration curve (AUC0-inf) 1036 pg•h/mL following SC administration.
If he injected with single exenatide LAR (Bydureon) dose (0.8 mg of exenatide but in sustained release or controlled release form) he has average Cmax (pg/mL) 64.3 (38.3-107.8) and AUC (0-8 hours) (pg*h/mL) 187.6 (133.7-263.3).
It's already clearly that single SC injection of BYETTA (10 mcg immediate release) creates much higher plasma level of exenatide (3-4 times higher) than BYDUREON (0.8 mg) single SC injection of exenatide (sustained release).
Just compare:
10 mcg BYETTA Cmax 211 pg/mL ; (AUC0-inf) 1036 pg•h/mL
0.8 mg BYDUREON Cmax 64.3 pg/mL (38.3-107.8); AUC (0-8 hours) 187.6 (133.7-263.3) pg•h/mL
In this case what FDA wants from AMLN?
Inject healthy volunteers with what dose of BYETTA? May be 0.8 mg of exenatide (as BYDUREON contains)?
But it will definitely kill these people.
"In a clinical study of BYETTA, three patients with type 2 diabetes each experienced a single overdose of 100 mcg SC (10 times the maximum recommended dose). Effects of the overdoses included severe nausea, severe vomiting, and rapidly declining blood glucose concentrations. One of the three patients experienced severe hypoglycemia requiring parenteral glucose administration. The three patients recovered without complication."
BYETTA never had any "cardiac safety concerns" and the study "Electrophysiological Effects of a Single 10 mcg Dose of Exenatide on the 12-Lead Electrocardiogram QT Interval in Healthy Subjects" was done by Eli Lilly and Amylin Pharmaceuticals, Inc. in 2008.
Primary Outcome Measures:
To determine, in healthy subjects, that a single 10 mcg dose of exenatide does not differ from placebo in the mean change from predose in 12-lead ECG correct QT (QTc) interval measurements
Estimated Enrollment: 80
Study Start Date: April 2008
Study Completion Date: July 2008
Primary Completion Date: July 2008 (Final data collection date for primary outcome measure)
http://clinicaltrials.gov/ct2/show/NCT00672399?term=Amylin+QT&rank=1
Sure, that the results were good.
So, this another through QT (tQT) study demanded by FDA is just nonsense.
Especially with this condition: "exposures of exenatide higher than typical therapeutic levels of BYDUREON"
I hoped that FDA commissioners are educated scientists and they know that SR (sustained release) or CR (controlled release) formulations are designed to provide much less plasma therapeutic levels of drug than immediate release formulation (to avoid sharp Cmax spikes in patient blood).
And actually BYDUREON single injection provides much less exenatide plasma level than BYETTA single injection (PK/PD data is here).
Conclusion: so, meet our new FDA, incompetent and may be corrupted. In case of AMLN they used "cardiac safety " as just a reason to postpone great new diabetes drug for at least 1.5-2 years. I will not be surprised if Europe and Japan approve BYDUREON first.
I'm not excluded the possibility that some "rat" inside FDA is working for Novo or other Amylin rivals. Novo is more probable, because BYDUREON after approval will just kill NVO Victoza sales.
So, Novo desperately needs additional time to prepare good and strong answer for BYDUREON. What the "silver bullet" they have against BYDUREON? They know that decrease in needle jabs is OK, but the best way is to avoid these needles at all.
Yes, NVO "silver bullet" is ORAL GLP-1. They already finished 2 phase I trials for this revolutionary diabetes drug.
http://clinicaltrials.gov/ct2/show/NCT01037582
http://clinicaltrials.gov/ct2/show/NCT01028404
I will write some comments about NVO oral GLP-1 later.
And I don't understand today AMLN panic sell off. Byetta is still very good diabetes drug. The patent 5,424,286 for BYETTA will expire 01-Dec-16 (or later)
http://www.uspto.gov/patents/resources/terms/156.jsp
AMLN net product sales for the quarter ended September 30, 2010 were $154.0 million. Unaudited net product sales for the quarter include $132.4 million for BYETTA® (exenatide) injection and $21.6 million for SYMLIN® (pramlintide acetate) injection.
http://finance.yahoo.com/news/Amylin-Pharmaceuticals-prnews-623432677.html?x=0&.v=1
AMLN pps must be at least in $15-20 range even without Bydureon approval because nothing happened with current Byetta sales.
And actually CRL is not rejected Bydureon. FDA asked about DURATION-5 trial results, but this trial is completed. tQT study is also short. So, finally Bydureon will be approved (definitely before Byetta patent expiration). So, I bought today additional 6k AMLN at average $9.95. Will keep it before pps will bounce back to normal range i.e. $15-18. Normal for company with annual net product sales >$600M.
Long release versus instant release
Byetta has already been available as a twice daily injection. How does it compare to the once weekly form? First, the most important note is that once weekly dosing is a lot easier than twice daily. Especially since Byetta is injected. Some studies have shown superior results achieving target levels for the long release form than the instant release. In one, for instance, 77% of those on long release achieved target HbA1c levels, while only 61% of those on instant release did. One study showed that people thought instant release and long release were about as effective. That study, however, showed that people rated long release as significantly more convenient than instant release.
Trial or other data
Jun 10: DURATION -3 results published in the Lancet (2010;375:2234-43). In the 26-week, open-label, study 456 patients were randomly allocated to 2mg once weekly exenatide or insulin glargine to target glucose range 4-5.5mmmol/L, plus existing oral treatments, metformin or metformin + sulphonylurea. Change in HbA1c was greater in patients taking exenatide (−1·5%, SE 0·05) vs those taking insulin glargine (−1·3%, 0·06; treatment difference −0·16%, 0·07, 95% CI −0·29 to −0·03). 5% vs 1% of patients respectively, discontinued participation because of AEs (p=0·012). Exenatide resulted in weight loss rather then weight gain. A planned extension period (up to 2·5 years´ duration) is in progress.
Jun 10: Top-line results from DURATION-4 reported. The 26-week study compared BYDUREON monotherapy to sitagliptin, pioglitazone or metformin in 820 patients with T2DM. Study participants were not not on any diabetes therapy when they entered the study. Patients randomized to BYDUREON experienced a reduction in A1C of 1.5% vs 1.2%, 1.6% and 1.5% for the oral hypoglycaemics, respectively. All groups, except the sitagliptin group, achieved an average A1C of <7% by study end. Average weight changes in the four groups were -4.5, -1.7, +3.3 and -4.4lb, respectively. >80% of patients in all treatment arms completed the study. There were no major hypoglycaemia events in any treatment group. The most frequently reported adverse events among BYDUREON users were nausea (withdrawal rate <1%) and diarrhea.
Apr 10: The FDA has raised concerns that Bydureon may be linked to increased cancer risk; data on IV dosing and the once-weekly formulation “seem to give a similar signal” as cancers seen in rodent studies of liraglutide. A statistically significant increase in thyroid cancers was seen in female rats given doses 25 times normal human doses. During the review of liraglutide (which was delayed by 10 months) the FDA said thyroid tumours may be common with all extended-release GLP-1 analogs.
Dec 09: positive results reported from a head-to-head study (DURATION-5) of exenatide once weekly vs exenatide twice daily, in 250 patients with type 2 diabetes not adequately controlled on other agents. After 24 weeks of treatment, patients had an HbA1C reduction from baseline of 1.6% vs 0.9% respectively and achieved a mean A1C of 7.1% vs 7.7%. Both treatment groups achieved statistically significant weight loss by the end of the study, with an average loss of 5.1 vs 3lbs respectively. Around 80% of patients completed the study. The most frequently reported AE in both groups was nausea (14% with weekly vs 35% with twice daily injections)
Jul 09: Company announced results from DURATION-3, a 26 week study in 467 patients with type 2 diabetes which compared once-weekly exenatide to Lantus. Patients had previously failed on metformin alone or in combination with sulfonylureas. Patients on exenatide achieved a mean A1C of 6.8% vs 7.0% in those on Lantus. Exentaide was associated with a lower incidence of hypoglycaemia and a mean weight loss of 5.8lb (2.6 kg) after 6 months vs with a weight gain of 3.1 lb (1.4 kg) with Lantus.
Jun 09: Two-year efficacy data from the DURATION-1 trial were presented at the ADA-2009 meeting. Reductions in A1C of 1.7% and FPG of 40mg/dL were maintained after two years of treatment. 65% of patients achieved an A1C of ≤7%. Body weight was reduced, patients losing an average of 5.8lbs. Serum lipid profiles were improved, and there was a reduction in systolic blood pressure.
Apr 09: Amylin and Lilly are to develop a pen device with a dual chamber cartridge configuration for exenatide once weekly. It will enable patients to mix and administer the preparation from a pre-filled pen device, instead of the syringe and vial currently used in trials.
Apr 09: Company state that no cases of thyroid cancer have been documented in clinical trials of Byetta or long-acting Byetta, following the revelation of such a link with liraglutide in animal studies at a recent FDA advisory panel meeting. Nine cases of ´spontaneous´ thyroid cancer have been reported in the approximate 1 million patients taking Byetta, according to FDA records, but none of these tumours were fatal nor of the type under FDA scrutiny with liraglutide
Apr 09: positive results reported from DURATION-2 – a 26-week study vs maximum doses of sitagliptin (100mg/d) or pioglitazone (45mg/d) in 491 patients on metformin. Patients on exenatide once weekly had a reduction in HbA1C of 1.7% vs 1% for sitagliptin and 1.4% for pioglitazone (statistically signif in favour of exenatide). At the end of the study patients on exenatide weighed an average of 14lbs less than patients on pioglitazone and 4lbs less than patients on sitagliptin.
Mar 09: A company meta-analysis of primary cardiovascular events (cardiovascular mortality, myocardial infarction, stroke, hospitalization for acute coronary syndrome and revascularization procedures) across controlled clinical studies of three months or greater, from the exenatide database, showed no increased cardiovascular risk. The relative risk of cardiovascular events in exenatide-treated patients vs. controls, was 0.70 [95%CI 0.38 - 1.31]. This finding will be used to support the cardiovascular safety of exenatide once weekly. (Results from DURATION-1, an open label study in 295 patients aged ≥16 years with type 2 diabetes mellitus, indicte that a new once-weekly formulation (2mg) of exenatide is non-inferior to twice daily formulation in improving glycaemic control. At 30 weeks, the mean reduction in HbA1c was -1.9% (SE 0.1%) in the once-weekly group compared to -1.5% (0.1%) in the twice daily group (mean difference -0.33; 95% CI −0.54% to −0.12%; p=0.0023). More patients receiving once-weekly exenatide achieved an HbA1c of 7% or less (77% vs 61% respectively, p=0.0039). Bodyweight decreased to similar extent in both groups over the trial period. Study published in the Lancet
Mar 09: DURATION-5, a new phase 3b study is starting in which patients with type 2 diabetes will use exenatide once weekly commercial-scale drug product in its final commercial configuration. This randomized, 26-week, open-label study in approximately 240 patients is designed to show superiority of exenatide once weekly compared to BYETTA, support regulatory submissions outside the US and provide additional controlled clinical data on the commercially manufactured product.
The DURATION trial programme is designed to demonstrate superiority of exenatide once weekly over other medications.
http://www.ukmi.nhs.uk/applications/ndo/record_view_open.asp?newDrugID=4704
March 29, 2011 by BiotechInvest
I think that current AMLN price ($10 range) and Market Cap ($1.54B) are absurdly low. Are biotech investors are blind and deaf? Nobody mentioned last AMLN news?
Amylin Says Once-Monthly Exenatide Showed Positive Results In Mid-Stage Study
Amylin Pharmaceuticals, Inc. (AMLN) announced Thursday positive results from a mid-stage study of a once-monthly subcutaneous injection of exenatide for the treatment of type 2 diabetes. Amylin is developing the extended-release formulation of exenatide with Eli Lilly and Co. (LLY) and Alkermes, Inc. (ALKS).
San Diego, California-based Amylin reported results from a 121-patient, phase 2 study, which assessed the efficacy, safety and tolerability of three different doses of exenatide once monthly.
The randomized open-label study was conducted on adults with type 2 diabetes who were not achieving adequate glucose control using diet and exercise alone or with a stable regimen of metformin, Actos, or both.
One arm of the study assessed exenatide once weekly, for which the proposed brand name is Bydureon. The other arm investigated three different doses of exenatide once monthly. Both formulations are subcutaneous injections of exenatide. Bydureon and exenatide once monthly are not currently approved by any regulatory agency.
Subjects were randomized to receive either 2 mg weekly subcutaneous injections of Bydureon or subcutaneous injections of exenatide once monthly at a low, medium or high dose, each administered once every four weeks, for a total of 20 weeks.
After twenty weeks of treatment, on a total of five injections, patients in exenatide once-monthly demonstrated reduction in average blood sugar. The reduction ranged between 1.3 and 1.5 percentage points from baseline. In the once-weekly Bydureon treatment arm, the reduction was 1.5 percentage points.
More than 90 percent of patients overall completed the study. The most common adverse events among the exenatide once monthly treatment groups were headache and nausea.
The extended release formulation of exenatide is based on the same Medisorb microsphere technology used in Bydureon. Exenatide is the active ingredient in Byetta exenatide injection, which is given twice daily.
Byetta is an injectable prescription medicine that may improve blood sugar control in adults with type 2 diabetes mellitus, when used with a diet and exercise program. Based on post-marketing data, Byetta has been associated with acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis.
Byetta was the first glucagon-like peptide-1 or GLP-1 receptor agonist to be approved by the FDA. GLP-1 improves blood sugar after food intake through multiple effects that work in concert on the stomach, liver, pancreas and brain.
Christian Weyer, M.D., senior vice president, research and development, Amylin Pharmaceuticals said, "As innovators in the treatment of type 2 diabetes we brought the first GLP-1 product, BYETTA, to patients. We are now developing once-weekly and once-monthly formulations of exenatide to expand patient choices for improving glycemic control."
Weyer added, "Based on the encouraging results of this study, we plan to proceed with regulatory interactions to outline the next steps for this important program."
Disclosure: I opened long AMLN positions today. AMLN pps is strongly undervalued now and I think that $10 is a good entry point for long-term positions.
April 13, 2011 by BiotechInvest
I bought AMLN (again) and now waiting for this trial results:
A Safety Study to Assess the Effects of Therapeutic and Supratherapeutic Exenatide Concentrations on QT Interval in Healthy Subjects
Primary Outcome Measures:
* The mean change from predose in 12-lead electrocardiogram corrected QT interval. [ Time Frame: From baseline up to and including 3 days post initial dose during each crossover treatment period. ] [ Designated as safety issue: Yes ]
Secondary Outcome Measures:
* To assess the pharmacokinetic-pharmacodynamic relationship between plasma concentration of exenatide at steady state and its effects, if any, on the QTc interval. [ Time Frame: From baseline up to and including 3 days post initial dose during each crossover treatment period. ] [ Designated as safety issue: Yes ]
* The safety and tolerability of exenatide as assessed by the incidence, nature and severity of adverse events. [ Time Frame: From baseline up to and including 3 days post initial dose during each crossover treatment period. ] [ Designated as safety issue: Yes ]
Estimated Enrollment: 100
Study Start Date: February 2011
Estimated Study Completion Date: June 2011
Estimated Primary Completion Date: June 2011 (Final data collection date for primary outcome measure)
FDA asked AMLN about this trial in CRL and in such way delayed Bydureon approval at least for 1 year. The trial results will be released in June-July 2011, so PDUFA for Bydureon will be in 2012. NVO should thanks FDA for Bydureon delay because NVO drug Victoza won US diabetes market during this time and NVO pps added +30%.
Is Victoza safe?
Possible thyroid tumors, including cancer
During the drug testing process, the medicine in Victoza® causedrats and mice to develop tumors of the thyroid gland. Some of these tumors were cancers. It is not known if Victoza® will cause thyroid tumors or a type of thyroid cancer called medullary thyroid cancer in people. If medullary thyroid cancer occurs, it may lead to death if not detected and treated early. If you develop tumors or cancer of the thyroid, your thyroid may have to be surgically removed.
Before you start taking Victoza®, tell your healthcare provider if you or any of your family members have had thyroid cancer, especially medullary thyroid cancer, or multiple endocrine neoplasia syndrome type 2 (MEN 2). Do not take Victoza® if you or any of your family members have medullary thyroid cancer, or if you have MEN 2. People with these conditions already have a higher chance of developing medullary thyroid cancer in general and should not take Victoza®.
While taking Victoza®, tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath. These may be symptoms of thyroid cancer.
Will patients sue NVO if thyroid cancer really happen in people who are taking Victoza each day?
Of course finally 1-weekly injection will win 1-daily injection and AMLN pps will be again >$20-25. But many biotech investors lose money because this FDA stupid сoncern about safety. They easy approved Victoza and sent CRL about Bydureon that was never linked to any cancers.
April 16, 2011 by BiotechInvest
Well, finally Europe showed more wisdom than USA.
"Potential heart problems? We don't see no stinking potential heart problems" -- a paraphrase of the Committee for Medicinal Products for Human Use of the European Medicines Agency.
Bydureon, has been recommended for approval by the European Medicines Agency.
According to Reuters, recommendations from the European Medicines Agency are normally endorsed by the European Commission within months i.e. already in 2011 diabetes patients in Europe will have the possibility to do 1-weekly injection of GLP-1 analog (Byetta) in extended release (ER) form. Less injections, less pain, 7 times less injection sites (Fewer Needle Pricks for the Europeans).
It's clearly that Bydureon will crash NVO Victoza sales in Europe (at least 7 times) and then in China (after Bydureon approval in China). So, before Q4 AMLN pps should return to $20-25 range.
And may be it's a good time to short this "bloated pig" NVO?
But I like NVO science and innovations and will not do it (just part of my credo). NVO has a strong "trump card" against Bydureon - oral "Victoza" (GLP-1 analog that has structure similar to liraglutide but it has much more longer half-life in blood > 24 hours). NVO already tested this oral form of "Super Victoza" (NN9924 oral) in Phase I trial. But Novo Nordisk prefers keep this program in secret and only modestly said at its website: "Our ambition is to develop break-through products with orally available versions of insulin and GLP-1".
"NN9924
:Type 2 diabetes
:Phase 1
Novo Nordisk is developing an oral GLP-1 to increase the convenience of GLP-1 treatment of type 2 diabetes.
To address the key challenges relating to oral delivery of GLP-1 Novo Nordisk has designed a GLP-1 analogue, which is more resistant to enzymatic degradation. Novo Nordisk has further established two separate partnerships with Emisphere and Merrion, both having developed unique formulation technologies facilitating GLP-1 absorption from the gut.
Company announcements
No company announcements matching the search criteria
Press releases
Novo Nordisk starts phase 1 trial with long-acting oral GLP-1 analogue (13 Jan 2010)
Read more press releases
Scientific abstracts
No scientific abstracts matching the search criteria"
And it will take 4-5 years more to bring this drug on diabetes market. After this diabetes patients will forget about pain injections (even 1-weekly) and will take 1 tablet per day of "Super Victoza" (not real name of this drug but if NVO will take it I will be flattered). Sure that NVO pps will at $200 range at this time (if they not split stock before).
Disclosure: I have AMLN positions (may be will add more). I don't have NVO positions and will not have them in next 2-3 years.
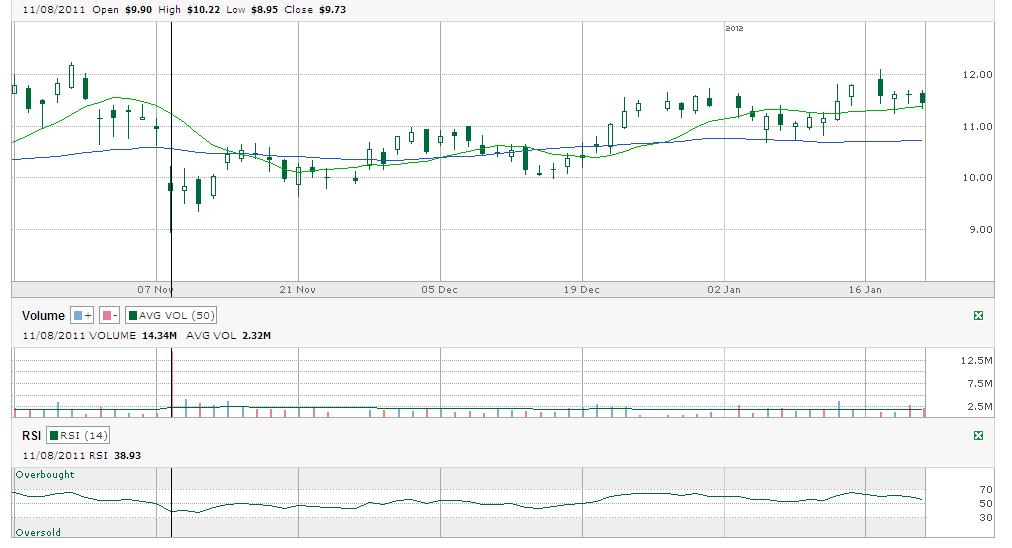
01/21/12 by BiotechInvest
Just 1 week before next (already third) AMLN PDUFA. Everybody is asking just one question: will FDA crash AMLN again with some stupid CRL?
And as a result of such possible scenario Amylin was downgraded to Sell from Neutral at MKM Partners Price target is $8.50.
And "speaking of discouraging news, the analysts at Rodman & Renshaw recently downgraded shares of Amylin Pharma. Arguing that Eli Lilly's (NYSE: LLY ) termination of a development agreement with the company "places a heavy financial burden" on Amylin, the analyst counsels investors to sell the stock.
But what if FDA approve AMLN diabetes drug?
AMLN Market Cap: 1.67B and it's main competitor (NVO) Market Cap: 67.12B
Novo Nordisk A/S, the world’s biggest maker of insulin, said first-quarter profit rose 23 percent, beating estimates, as demand for its newest treatment, Victoza, helped offset government price cuts.
Net income climbed to 4.07 billion kroner ($810 million) from 3.32 billion kroner, as Victoza sales grew threefold, the Bagsvaerd, Denmark-based drugmaker said. That beat the average estimate of 3.91 billion kroner in a Bloomberg survey of 14 analysts. Sales rose 15 percent to 15.7 billion kroner.
Novo Nordiskbegan selling Victoza in 2009 and planned this year to market the treatment in several emerging markets. Sales of the drug, which mimics a hormone called GLP-1 and stimulates natural insulin production, climbed to 1.1 billion kroner, beating an average estimate of 1.01 billion kroner in a Bloomberg survey.
Victoza likely will be a blockbuster, with sales of at least $1 billion, “at the turn of this year,” Chief Financial Officer Jesper Brandgaard said today. The treatment helped boost Novo Nordisk’s share of the total global diabetes market, by value, to 24 percent from 23 percent, he said.
And real question is here: will NVO allow weekly (and may be later monthly) Bydureon to compete with daily Victoza and cut at least $1 billion annual sales?
May be it's better for NVO to buy AMLN now for 100% premium (i.e. just for $3.4B)?
Why analysts keep silence about this possibility after Bydureon approval? And who is buying tens million of AMLN shares starting from November when they created this "artificial panic"?
Disclosure: I have AMLN positions.
01.30.12 by BiotechInvest
One asked me: will I sell AMLN now after approal? My answer is yes, but only for >$30
Bydureon potential is huge (bigger than JAZZ Xyrem and still unreal sales of "oral only" HCV drug of VRUS ) so if Big Pharmas are still thinking about AMLN buyout opportunity it's their problems.
Shares Out.146.3M
% Held by Inst 83.73% = 121M
$14.26 2.12 (17.46%) Today Volume 21,001,023 (Heavy) = 14% (100 - 14 = 86) i.e. almost all private investors sold AMLN today. To whom? Funds bought everything. And now they have 100% AMLN shares.
They will wait now for AMLN buyout (>$30 per share) and I will wait too.
Question is who is a buyer for AMLN? NVO, Takeda, Sanofi... Japan or Europe?
Carl Icahn (>10% owner) will be glad to sell AMLN now (BTW some www.mffais.com said that recently Icahn Carl C Et Al bought 14,381,925 shares of Amylin Pharmaceuticals Inc (AMLN), prior they they did not have any shares. It seems like that he bought AMLN at $9 range and already has a huge gain.
02.03.12 by BiotechInvest
I sold AMLN yesterday to avoid any potential losses induced by possible dilution, drop of Byetta sales (because of Bydureon), absence of buyer and partners (look at SVNT when they said that there were no buyers).
Next targets are STEM (already bought) and VVUS (will buy today).
ABG (always be green)!