Hot Watch List (comments).
CARA (buy) 10/07/15 updated to STRONG BUY 04/09/18 updated to STRONG BUY
12/24/14 by BiotechInvest
Cara Therapeutics, Inc., a clinical-stage biopharmaceutical company, focuses on developing and commercializing chemical entities designed to alleviate pain by selectively targeting kappa opioid receptors. Its lead product candidate includes intravenous CR845, which has completed Phase II clinical trials for the treatment acute postoperative pain in adult patients. The company is also developing Oral CR845 that has completed Phase I clinical trial for the treatment of moderate-to-severe acute and chronic pain; and CR701, which is in preclinical trial for treating neuropathic and inflammatory pain. Cara Therapeutics, Inc. was founded in 2004 and is based in Shelton, Connecticut.
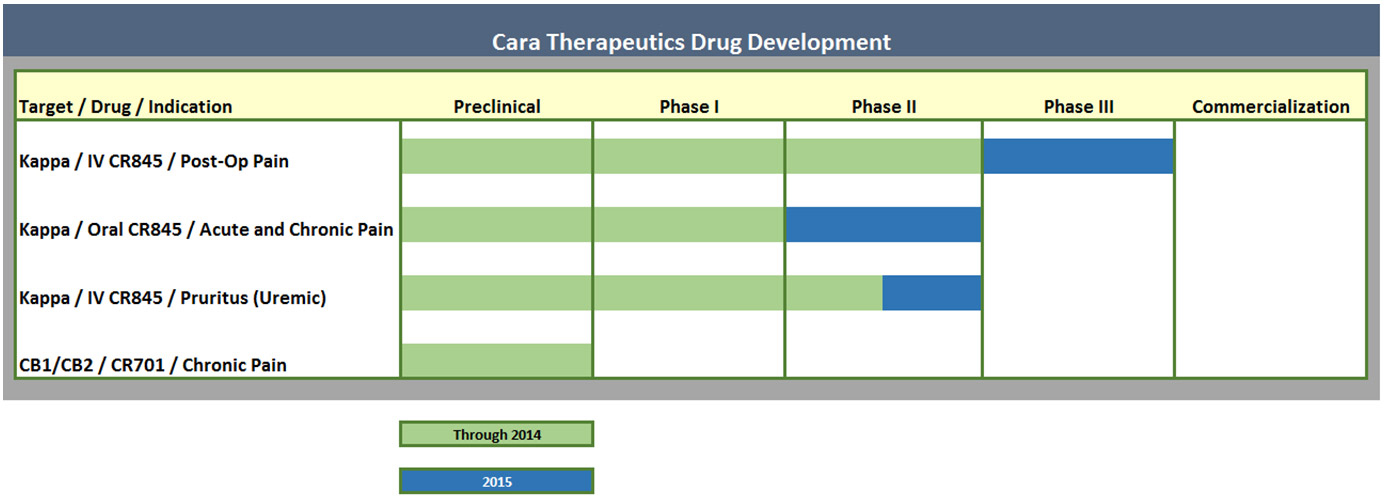
Look at Cara pipeline: it's mostly about one compound CR845.
CR845
CARA POSSESSES PERIPHERALLY-SELECTIVE MOLECULES THAT INTERACT WITH KAPPA OPIOID RECEPTORS PRESENT ON PERIPHERAL, PAIN-SENSING NERVES.
These compounds exhibit potent analgesic and anti-inflammatory properties in animals. Unlike currently marketed opioids, these new compounds do not produce inhibition of intestinal transit (ileus), do not induce life-threatening respiratory depression, nor do they elicit signs of addiction or euphoria in animal models.
CR845 belongs to this pharmacological class of compounds; it is a potent peripheral kappa opioid receptor agonist with high selectivity over other opioid receptors. Such compounds are thought to have the potential to provide pain relief (peripheral opioid analgesia) without producing significant CNS side effects.
Pain killer without side-effects and addiction?
Pain killers market is huge (i.e. billions $) and CARA cap is just $223M (at pps $9.80). If phase III of CR845 for trial post-operational pain is succeed will Big Pharma acquire CARA? Sure YES. May be $1B is very modest price for biotech with new pain killer that can be delivered not only via IV but orally also:
Enteris BioPharma's Formulation Technology Enables Oral Delivery of Cara Therapeutics' CR845 Peptide for Acute and Chronic Pain in Phase 1a / 1b Trial
Cara Therapeutics reported that all tested tablet strengths of Oral CR845 were pharmacologically active, safe and well tolerated after single and multiple dose administration
PR Newswire Enteris BioPharma, Inc.
December 9, 2014 8:10 AM
BOONTON, N.J., Dec. 9, 2014 /PRNewswire/ -- Enteris BioPharma, Inc., an industry leader in oral peptide delivery, congratulates Cara Therapeutics (CARA) on the successful completion of the Phase 1a / 1b clinical trial of its peripherally-selective kappa opioid agonist, CR845, for the treatment of acute and chronic pain. Cara's tablet formulation of CR845 utilizes Enteris' proprietary oral delivery technology.
The Cara study showed a range of maximum plasma concentrations of CR845 bracketing the concentrations seen in previous Phase 2 studies with IV CR845, equivalent systemic levels of CR845 in oral and IV administration, dose linearity over a 100 fold range (from 0.1mg to 10mg) and that all doses studied were biologically active, safe and well tolerated.
Brian Zietsman, President and CFO of Enteris BioPharma, commented: "We congratulate Cara Therapeutics on the successful completion of its Phase 1 study of oral CR845. As Cara Therapeutics seeks to expand the clinical development of CR845 beyond the treatment of acute pain in an institutional setting, oral delivery of CR845 will be the key to unlock this opportunity."
Oral pain killer without addiction and side effects is very valuable.
Conclusions: I'll buy CARA for long-term investment (1-2 years) in nearest future.
10/07/15 by BiotechInvest
CARA will become valuable acquisition target for Big Pharma after successful completion of phase 2-3 clinical trial A Study Evaluating the Overall Pain Relief and Safety of Intravenous (IV) CR845 in Patients Undergoing Abdominal Surgery
Estimated Enrollment: 600
Study Start Date: September 2015
Estimated Study Completion Date: June 2016
Estimated Primary Completion Date: April 2016 (Final data collection date for primary outcome measure)
Primary Outcome Measures:
A comparison of the change in Pain Intensity over time using the Numeric Rating Scale [ Time Frame: 24 hours ] [ Designated as safety issue: No ]
Using the Numeric Rating Scale (NRS) to quantify the difference between CR845-treated patients and placebo-treated patients.
Secondary Outcome Measures:
A study of the comparison of Post Surgical Nausea and Vomiting (PONV) scores [ Time Frame: 6 hours ] [ Designated as safety issue: No ]
Incidence of vomiting will be analyzed against placebo
A comparison of the number of rescue medication doses administered [ Time Frame: 24 hours ] [ Designated as safety issue: No ]
Count the number of rescue medication doses administered following surgery for the CR845 IV patients vs Placebo-treated patients
Patient Global Assessment of study drug [ Time Frame: 24 hours (or early termination) ] [ Designated as safety issue: No ]
Patients will complete a PGA (Patient Global Assessment) of the study drug.
Conclusions: CARA is STRONG BUY (at pps $15 and up to $20) in 2015-16
03/31/16 by BiotechInvest
It was disappointing news for CARA holders:
SHELTON, Conn., Feb. 25, 2016 (GLOBE NEWSWIRE) -- Cara Therapeutics, Inc. (NASDAQ:CARA), a biotechnology company focused on developing and commercializing new chemical entities designed to alleviate pain and pruritus by selectively targeting kappa opioid receptors, today announced that it has received oral notification from the U.S. Food and Drug Administration (FDA) that its adaptive pivotal trial of I.V. CR845 for postoperative pain has been placed on protocol clinical hold pending a pre-specified safety review. A protocol-specified stopping rule, based on elevated serum sodium levels greater than 150 mmol/L, was met during the first phase of the study, and a review of unblinded safety data has been undertaken by both Cara and the Independent Data Monitoring Committee (IDMC) in accordance with the protocol. The initial results of this safety data review show:
Out of 90 total patients dosed to date, four patients in the highest CR845 dose group (5 ug/kg) exhibited transient serum sodium levels equal to or greater than 150 mmol/L (mild-to-moderate hypernatremia). All four patients were asymptomatic and sodium levels resolved to normal levels (<146 mmol/L) within 24 hours post-dosing with standard fluid management. No patients in the other two dose groups — 2 ug/kg and 1 ug/kg — exhibited serum sodium levels greater than 150 mmol/L. The most common adverse events (> 5%) reported across treatment groups and placebo so far were nausea, hypernatremia, abdominal distension and procedural hypotension. All cases of abdominal distension and procedural hypotension were attributed to the surgical procedure and not to study drug. There were no cases of respiratory depression, no adverse events greater than Grade 1, and no CR845-associated serious adverse events have been reported. "We are working closely with the FDA to review patient safety data and resolve this issue in a timely manner," said Derek Chalmers, Ph.D., D.Sc., President and Chief Executive Officer of Cara Therapeutics. "We remain very confident in the overall efficacy and safety profile of I.V. CR845 in the treatment of postoperative pain, and are encouraged that there have been no serious CR845-related adverse events so far in our Phase 3 program. We look forward to continuing the study, pending FDA review, as we work to bring this novel class of therapeutics to patients in need of additional pain treatment options." The CLIN3001 study is a multi-center, randomized, double-blind, placebo-controlled, parallel-group adaptive design Phase 3 trial with repeated doses of I.V. CR845 or placebo administered both prior to and following abdominal surgery in male and female patients. The trial is enrolling up to 600 patients undergoing either hysterectomy, prostatectomy, hemi-colectomy or ventral hernia repair at 30 clinical sites within the U.S. Three doses of I.V. CR845 (1.0, 2.0 and 5.0 ug/kg I.V.) are being compared to placebo. The primary efficacy measure is the Change in Pain Intensity over the 24-hour postoperative period (AUC-24) using the patient-reported Numeric Rating Scale (NRS) score collected at pre-specified time points through 24 hours. Postoperative nausea and vomiting (PONV), which were reduced in previous clinical trials of I.V. CR845, are being evaluated as a secondary efficacy measure.
Is hold a real problem for CARA stock? Nope
CARA said:
"Out of 90 total patients dosed to date, four patients in the highest CR845 dose group (5 ug/kg) exhibited transient serum sodium levels equal to or greater than 150 mmol/L (mild-to-moderate hypernatremia). All four patients were asymptomatic and sodium levels resolved to normal levels (<146 mmol/L) within 24 hours post-dosing with standard fluid management. No patients in the other two dose groups — 2 ug/kg and 1 ug/kg — exhibited serum sodium levels greater than 150 mmol/L."
What is hypernatremia?
"Hypernatremia is a common electrolyte problem and is defined as a rise in serum sodium concentration to a value exceeding 145 mmol/L.[1, 2] It is strictly defined as a hyperosmolar condition caused by a decrease in total body water (TBW) relative to electrolyte content. Hypernatremia is a “water problem,” not a problem of sodium homeostasis."
Patients who develop hypernatremia during the course of hospitalization have an age distribution similar to that of the general hospital population. In both patient groups, hypernatremia is caused by impaired thirst and/or restricted access to water, often exacerbated by pathologic conditions with increased fluid loss.
Correction of hypernatremia requires the administration of dilute fluids to both correct the water deficit and replace ongoing water losses, and also, when appropriate, interventions to limit further water loss.
Why CR845 high doses induces hypernatremia? The answer is simple: CR845 is a peripherally acting kappa opioid receptor agonist. And it is known that "kappa-opioid receptor agonists inhibit antidiuretic hormone secretion and promote water excretion in humans and experimental animals".
Thus, it is normal that kappa opioid receptor agonists have potent aquaretic effect (i..e. they are drugs that bind to vasopressin receptors in the renal collecting duct and promote an excretion of solute-free water). So, kappa-opioid receptor agonists can be even useful for the treatment of water retention and dilutional hyponatremia in cirrhosis.
So, the increasing of plasma sodium concentration (or hypernatremia) is common effect for kappa-opioid agonists:
J Hepatol. 2000 Jan;32(1):38-42.
Aquaretic effects of niravoline, a kappa-opioid agonist, in patients with cirrhosis.
Gadano A1, Moreau R, Pessione F, Trombino C, Giuily N, Sinnassamy P, Valla D, Lebrec D.
Abstract
BACKGROUND/AIMS:
In patients with cirrhosis, decreased renal water excretion is a common complication. Niravoline (RU51599), a kappa-opioid receptor agonist, has been shown to induce an aquaretic response. The aim of this study was to evaluate the aquaretic effect and tolerance of niravoline in patients with cirrhosis.
METHODS:
Biochemical tests and hemodynamic values were determined before and 1, 2, 3 and 24 h after niravoline administration at doses ranging from 0.5 to 2 mg iv in 18 patients with cirrhosis.
RESULTS:
Diuresis significantly increased in the first hour from 64+/-9 to 146+/-31 ml/h, and returned to basal values after 3 h. Free water clearance also significantly increased, reaching the positive range at 1 h. Plasma osmolality significantly decreased at 2 h (from 290+/-4 to 286+/-4 mOsm/kg). Plasma sodium concentrations increased significantly at 3 h (from 133+/-1 to 134+/-1 mEq/l). Heart rate and arterial pressure did not change. The highest doses (1.5 mg or 2 mg) induced personality disorders and mild confusion within 2 h. These effects reversed completely within 8 h.
CONCLUSION:
This study shows that niravoline administration induces an aquaretic response and is well tolerated, at moderate doses, in patients with cirrhosis. Thus, moderate doses of niravoline may be useful for treating patients with cirrhosis and water retention.
Conclusions: mild-to-moderate hypernatremia) induced by highest CR845 dose (5 ug/kg) is not a big problem for CARA drug. After FDA lift hold for Phase 3 trial CARA pps may easy jump to $16 and higher.
"Administration of kappa opioids decreases the urinary excretion of sodium and urine osmolality. In conscious rats, i.c.v. administration of U-50,488H (1µg total), a selective kappa opioid agonist, increased urine flow rate and decreased urinary sodium excretion. In these studies, U-50,488H also produced an increase in efferent renal sympathetic nerve activity during the duration of the antinatriuretic response. Prior bilateral renal denervation prevented the decrease in urinary sodium excretion produced by i.c.v. U-50,488H. Therefore, it was concluded that central kappa opioids produce antinatriuresis via an increase in sympathetic outflow to the kidneys."
antinatriuresis (ant″i-nā″trē-yŭ-rē′sĭs) [ anti- + natriuresis]
A decrease in the excretion of sodium in the urine.
04/20/16 by BiotechInvest
Cara Therapeutics Announces Removal of FDA Clinical Hold on CLIN3001 Postoperative Pain Trial for I.V. CR845
Patient recruitment expected to resume in May
Study to evaluate two doses of I.V. CR845 versus placebo
The results of this safety data review confirmed that increases in serum sodium levels in CR845-treated patients beyond the normal range were dose-dependent and asymptomatic with the lowest frequency of events found in the 1 ug/kg I.V. CR845 group. Based on this safety review and an analysis of efficacy trends, the study will continue as a three-arm trial testing two doses of CR845 (1 ug/kg and 0.5 ug/kg) versus placebo.
Actually it's very good sign: it means that doses 0.5 ug/kg (new one) and 1 ug/kg are already very effective.
CARA started Ph3 with three doses of I.V. CR845 (1.0, 2.0 and 5.0 ug/kg I.V.) and now study will continue as a three-arm trial testing two doses of CR845 (1 ug/kg and 0.5 ug/kg) versus placebo.
FDA put hold because:
"Out of 90 total patients dosed to date, four patients in the highest CR845 dose group (5 ug/kg) exhibited transient serum sodium levels equal to or greater than 150 mmol/L (mild-to-moderate hypernatremia). All four patients were asymptomatic and sodium levels resolved to normal levels (<146 mmol/L) within 24 hours post-dosing with standard fluid management. No patients in the other two dose groups – 2 ug/kg and 1 ug/kg – exhibited serum sodium levels greater than 150 mmol/L."
Now after data analysis they know:
"The results of this safety data review confirmed that increases in serum sodium levels in CR845-treated patients beyond the normal range were dose-dependent and asymptomatic with the lowest frequency of events found in the 1 ug/kg I.V. CR845 group."
Question is why CARA removed dosing with 2 ug/kg that was safe and never induce increasing of serum sodium level? And why they added 0.5 ug/kg?
Because all data were unblinded:
Derek Chalmers, Ph.D., D.Sc., President and Chief Executive Officer of Cara Therapeutics said: "Our unblinded analysis of the initial cohort of patients has identified interim efficacy signals for pain, supplemental opioid use and opioid-related side effects that support our dose selections.
Based on this safety review and an analysis of efficacy trends, the study will continue as a three-arm trial testing two doses of CR845 (1 ug/kg and 0.5 ug/kg) versus placebo.
Short conclusion: CARA drug is very effective pain killer and will work at 0.5-1 ug/kg without any significant side effects. With three-arms we will see the results sooner i.e. before Estimated Primary Completion Date: December 2016
04/27/16 by BiotechInvest
One more time about continuation of CARA Ph3 with doses 1 and 0.5 ug/kg
What was actual goal of this trial?
The answer is “overall Pain Relief and Safety of Intravenous (IV) CR845
drug CR845 will be administered intravenously prior to surgery, and at specific time intervals post surgery: CR845 IV 1 mcg/kg will be administered as an IV bolus one hour prior to anesthetic induction for surgery, again within 30 minutes of the patient being considered stable in the post operative recovery room, then 2 hours following that Baseline dose. Subsequent dosing will be administered at 6, 12 and 18 hours after the Baseline dose. Antinausea rescue medication (ondansetron 4 mg IV) may be requested, as well as analgesic rescue medication (morphine 5mg IV), post surgery, as needed.
Drug: Placebo IV
Placebo IV will be administered as an IV bolus one hour prior to anesthetic induction for surgery, again within 30 minutes of the patient being considered stable in the post operative recovery room, then 2 hours following that Baseline dose. Subsequent dosing will be administered at 6, 12 and 18 hours after the Baseline dose.
Antinausea rescue medication (ondansetron 4 mg IV) may be requested, as well as analgesic rescue medication (morphine 5mg IV), post surgery, as needed.
During the Treatment and Observation Period, pain intensity scores will be obtained at specified time points and episodes of nausea or vomiting will be recorded. Upon request, patients may be provided with analgesic or anti-nausea rescue medication (restricted to morphine (if tolerated), and ondansetron, respectively) at any time after the Baseline dose of study drug is administered.
Definitely, main goal of CR845 administration is a decreasing of usage of analgesic medication like morphine by patients after abdominal surgery. Morphine is a narcotic; so many patients can become addictive after several injections. So, less injections of morphine is better.
"Currently, the most widely used drugs to treat pain after surgery are opiates, such as morphine. Morphine works mainly by activating one of several types of opiate receptors that control some of our pain sensation - the so-called mu opiate receptors. These receptors are located in many areas of the brain and also outside of the brain. By activating these receptors, morphine provides significant pain relief, but also causes side effects that limit its use. Some of these side effects include: respiratory depression or arrest (slowed or stopped breathing), sedation (a state of calmness or extreme relaxation), euphoria (an exaggerated feeling of physical and mental well-being), constipation, nausea, vomiting, and drug addiction. In order to avoid the side effects of morphine and other mu opiates, the present experimental drug CR845 was designed to work at a different type of opiate receptor - called kappa - that can also provide pain relief, by acting on sensory nerves outside the brain. CR845 was designed to penetrate the brain much less than other opiate drugs, which should result in pain relief similar to that of morphine, but with fewer side effects. Because CR845 activates kappa receptors instead of mu receptors, the side effects are different than with a morphine-type drug. In particular, kappa opiates, such as CR845, do not cause respiratory depression or arrest, euphoria, constipation, drug tolerance, physical drug dependence or drug addiction. For these reasons, CR845 may present a distinct advantage over other opiates that are currently used for pain relief and post-operative pain in particular.
Most important question for any biotech investor is: will phase 3 trial meet their primary goals? If you know answer you will become rich.
Primary Outcome Measures:
• A comparison of the change in Pain Intensity over time using the Numeric Rating Scale [ Time Frame: 24 hours ] [ Designated as safety issue: No ]
Using the Numeric Rating Scale (NRS) to quantify the difference between CR845-treated patients and placebo-treated patients.
Treated arm will get 6 injections of CR845 (one hour prior to anesthetic induction for surgery, again within 30 minutes of the patient being considered stable in the post operative recovery room, then 2 hours following that Baseline dose. Subsequent dosing will be administered at 6, 12 and 18 hours after the Baseline dose) i.e. with 1 ug/kg the entire dose for 80 kg patient will be 480 ug CR845.
At the same time placebo patients will have only with the same volume of buffer but with no active drug (just saline).
Question: who will be in stronger pain and ask about morphine injections (may be multiple ones) placebo patients or CR845-treated patients?
The answer is clear for everybody who had abdominal surgery at least one time: (placebo patient will practically immediately ask about analgesic rescue medication morphine 5mg IV) when he/she become conscious.
Some skeptics will say that Ph3 trial will fail primary goals because CR845 doses 0.5 and 1 ug/kg are too low to provide strong analgesic effect. It’s not true: 6 injections each 1 ug/kg will give CR845-treated patient with 80 kg weigh almost 500 ug of strong analgesic during 18 hours. It’s more than enough to decrease pain and decrease (if any) morphine injections during 18 hours. 0.5 ug/kg also will give 250 ug per 18 hours and also should decrease number of morphine injections.
Total morphine consumption in the 8-16 hour period after abdominal surgery for placebo patients will be much higher than for CR845-treated so CR845 will present a distinct advantage over other opiates that are currently used for pain relief and post-operative pain in particular.
07/07/2017 by BiotechInvest
I sold CARA before pps crash (see my tweet below):
BiotechInvest @Biotech_Invest Jun 27
06/27/17: sold CARA for a gain (results of Ph3 CR845 in Patients after Abdominal Surgery can be released soon, but risk of failure is high).
I hope that some of my followers took my advice and avoided big losses with CARA.
The reason was simple: I have some doubts that oral delivery of CR845 was optimized by CARA i.e. the bioavailability of oral CR845 is too low to have some strong effect on pain. For example it is 1% from 5 mg (5000 micrograms) - only 50 mcg will be delivered to patient body. With average weight 100 kg patient will receive only 0.5 mcg/kg i.e. very low dosage in comparison with Ph2 trial where CARA dosed patients with 8 mcg/kg and even 40 mcg/kg.
In current Ph3 A Study Evaluating the Overall Pain Relief and Safety of Intravenous (IV) CR845 in Patients Undergoing Abdominal Surgery they dose only 0.5-1 mcg/kg. So, if the highest dose is equal to oral 5 mcg Ph3 will also fail to meet primary goals. CARA pps can again crash 50-60% and investors will get huge losses.
Will continue monitor CARA but it's very unlikely that I'll buy CARA before Ph3 results release. It's too high risk of Ph3 failure.
04/09/18 By BiotechInvest
CARA is on a finish line for CR845 Ph3 in Patients Undergoing Abdominal Surgery. The probability of Ph3 success >80% so STRONG BUY for CARA.